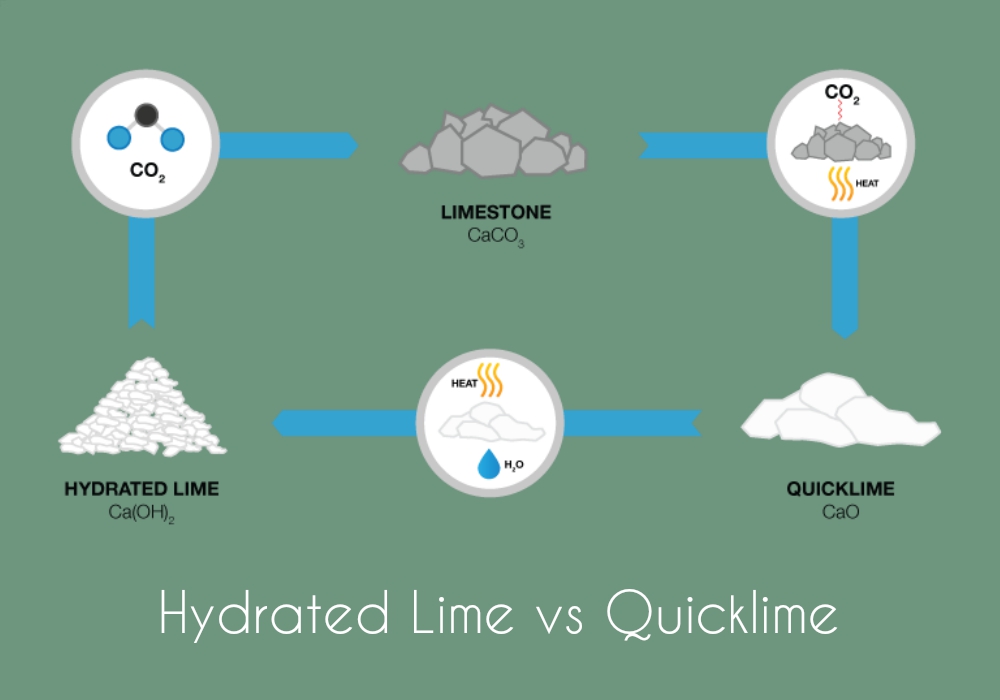
Differences Between Hydrated Lime and Quicklime
The term lime is often confused between two types – hydrated lime and quicklime. Both are calcium compounds but what varies amongst them is their chemical composition and their applications. Calcium in its hydrated state is known as calcium hydroxide, Ca(OH)2 and is commonly known as hydrated lime. In its pure form, calcium is known as calcium oxide, CaO; traditionally recognized as quicklime.
To compare based on density and reactivity, calcium hydroxide has light density (and is less reactive than calcium oxide. Hydrated lime (also known as slaked lime) is produced by adding water to the powdered form of quicklime, heating it in an oven and pulverizing it with water to get a powdered form of lime called hydrated lime. The density of calcium oxide is 65lb/ft³ whereas the density of calcium hydroxide is about 35lb/ft³.
Since quicklime has high reactivity, it is important to slake it as it has the capacity to generate heat at a higher temperature when exposed to water. On the other hand, hydrated lime doesn’t require neutralization and is safe to mix with water. This is why lime slurry has a traditional application to control the ph of water in the water supply system. One of the top uses of hydrated lime powder is soil rehabilitation that helps to significantly improve its engineering properties.
How to determine which type of lime to use?
The type of lime is selected for usage according to its process feed rate. The factor that determines suitability in a given process helps. This is based on the form of lime available versus the form of lime required.
For example, in a flue gas treatment that has a wide range of applications in the coal industry, cement industry, pollution & waste treatment sector or glass industry, the two categories of lime play different roles. Some industries that require flue gas treatment demand using slaked lime as a catalyst or filtrate whereas some others require quicklime for the same reason. Though the chemical compounds and the process feed rate are quite similar for each of these industrial usages, the difference in process and chemistry determines which type of use. To determine which type of lime slurries to use for a given system.
Uses of hydrated Lime vs Quicklime as per Equipment Type
The amount of lime a process of a given system requires can help in determining the type of lime slurries to use. If the system demands for large quantities, quicklime is a favorable option due to its high density. Although, for such processes, a lime slaker is used to prevent the hydrophobic reaction of CaO. The equipment generates Ca(OH)2 in a solution by mixing quicklime with water within a control temperature. The solution is called lime slurry. On the contrary, when a small to mid amount of lime solution is essential, hydrated lime is utilized. This is when the system uses simple equipment as the process doesn’t include any kind of exothermic reaction after water addition. For such processes that require hydrated lime, reaching out to hydrated lime suppliers in India can be helpful in meeting system requirements.